#157: Chytridiomycosis

Frog killed by chytridiomycosis. By Photo credit: Forrest Brem [CC BY 2.5], via Wikimedia Commons (cropped)
Impact of Chytridiomycosis
Since it began spreading in the 1970’s, Bd chytridiomycosis has driven many amphibian species to extinction and has caused severe declines in hundreds of others.ii A 2007 paper from the International Union for Conservation of Nature’s Species Survival Commission described chytridiomycosis as, “the worst infectious disease ever recorded among vertebrates in terms of the number of species impacted, and it’s propensity to drive them to extinction.” Sadly, that statement still holds true today. One reason why chytridiomycosis is so bad is that Bd can infect most of the world’s 6,000 or so amphibian species.iii However, the disease does not impact all amphibian species equally. Some frog populations experience 100% mortality from chytridiomycosis, while others are tolerant of the disease.iv Unfortunately, species that are specialized to small niches or have relatively small populations to begin with are the ones that are hit hardest by Bd.v Today, Bd can be found nearly everywhere that amphibians live.vi

Fire Salamander infected with Bsal chytridiomycosis (note the excessive black dots). By F. Pasmans (doi:10.1371/journal.ppat.1005251) [CC0], via Wikimedia Commons
Chytridiomycosis Symptoms
Bd chytridiomycosis is hard to diagnose from symptoms. These symptoms are caused by a variety of other diseases, making diagnosis difficult. Sometimes, frogs that die from Bd do not show any symptoms at all and just die. Symptoms of Bd infection include excessive sloughing of skin, skin discoloration, and abnormal behavior. As a result, a diagnosis of Bd chytridiomycosis requires laboratory testing.viii
Bsal chytridiomycosis, on the other hand, routinely produces observable symptoms. Diseased salamanders become lethargic and often develop large skin lesions. Salamanders that are in the early stages of infection often do not show any symptoms.ix
Pathogen Life Cycle
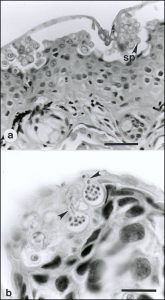
Chytridiomycosis infecting the skin of a frog. Arrows denote zoosporangia, bar = 35 µm. By Peter Daszak, Lee Berger, Andrew A. Cunningham, Alex D. Hyatt, D. Earl Green, and Rick Speare [Public domain], via Wikimedia Commons
The cyst then germinates and sends out a germ tube into a skin cell of its new amphibian host. The tip of the germ tube swells and the contents of the cyst are transferred to the swelling. This new thallus absorbs nutrients from its host cell and then may produce a thin protrusion called a rhizoid. This rhizoid serves essentially the same function as the germ tube. It swells at the tip and transfers the contents from the old thallus to the new thallus, thus allowing the chytrid to move from one cell to the next. Through this process, the pathogen can invade deep skin tissues.xi
When the chytrid has absorbed enough nutrients, it begins the reproductive phase of its life cycle. Once this phase begins, the thallus is converted into a spore-producing cell called a zoosporangium. During the first step in this cycle, nuclei inside the zoosporangium divide mitotically and the zoosporangium develops a thin tube that leads to the external environment. These discharge tubes remain plugged at the end until the spores are mature. Next, new cell walls develop within the zoosporangium and the nuclei are separated out into individual cells. These cells then develop flagella and become zoospores. Once all the spores are mature, the plug in the discharge tube dissolves and the zoospores are released. If the zoosporangium formed deep within the host’s skin, it will be pushed to the surface by developing skin tissue by the time it is ready to release its spores.xii
Pathogen Ecology
Bd and Bsal are remarkable because they are the only known chytrids to infect vertebrates.xiii They specialize in infecting skin that contains keratin, which amphibians have in abundance. However, immature amphibians like tadpoles are usually not bothered by the infection. This is because keratinized skin is present only around mouthparts in juveniles, which represents a very small amount of their total skin area. Unfortunately, juveniles are still susceptible to widespread infection once the metamorphose into adults.xiv
When there are no suitable hosts around, Bsal is able to survive by decomposing leaf litter. Switching to this alternative nutritional source allows Bsal to persist in the environment for long periods of time.xv Some observations suggest that Db also remains in the environment for a surprisingly long time. Nobody has observed Db to produce highly-resilient resting spores, so it is possible that Db can take advantage of a more normal chytrid habitat when its preferred hosts are not around. Some speculate that Db is able to survive in the absence of amphibians by either decomposing keratin debris as a saprobe or causing minor infections in alternate hosts.xvi Research into these possibilities has so far proved inconclusive. In a laboratory setting, Db was able to infect crawfish, which could then spread the infection to frogs. However, it was not established whether or not this occurs in the environment.xvii
The success of both Bd and Bsal is highly dependent upon temperature. Bd prefers temperatures from 17 to 25°C (63 to 77°F), while Bsal flourishes in cooler temperatures from 10 to 15°C (50-59°F).xviii Both grow much more slowly when outside their optimum temperature range, which causes seasonal variations in the prevalence of chytridiomycosis. For Db, frogs in temperate areas show a higher infection rate in the summer while frogs in the tropics are more likely to be infected during the winter.xix
Temperature stability is another factor that determines how successful Bd and Bsal will be. One study found that unpredictable changes in temperature increased stress in frogs and made it more difficult for them to fight of Bd. Climate change is currently driving increased variability in weather, so unpredictable temperature changes are becoming more common across the globe. Although it is impossible to measure the effect this is having on frogs in the real world, this research points to another way humans are helping drive the spread of disease.xx
How Chytridiomycosis Kills
In most species, a disease like chytridiomycosis would not be deadly. Amphibians, however, use their skin for more than just a protective coating. The skin of an amphibian also functions as a primary location for water, electrolyte, and gas exchanges. Consequently, skin infections impair an amphibian’s ability to regulate those exchanges, which can result in death.xxi Bd disrupts the function of the skin through both physical and chemical processes. As it grows, the fungus secretes proteins that break apart skin cells and inhibit the amphibian’s immune response. It also appears that the fungus actively inhibits the action of sodium/potassium pumps, which regulate electrolyte levels. Reduced ability to regulate water and electrolytes decreases the amount of water and sodium in the blood, which eventually causes cardiac arrest.xxii
Host Resistance

The American Bullfrog is resistant to chytridiomycosis and may help spread the infection. By Carl D. Howe (Carl D. Howe, Stow, MA USA) [CC BY-SA 2.5], via Wikimedia Commons
Resistance to Bd appears to be primarily moderated by innate mechanisms. These prime the immune system to effectively fight Bd. Additionally, they can be inherited and have allowed some frog species to increase in numbers despite continued infection with Bd. Unfortunately, species that do not have innate resistance cannot evolve such resistance. In the frog species that have been studied, there are only a few examples of adaptive immune resistance. It appears that the immune system of amphibians is not easily able to mount an adaptive response (this is the kind of immune response that that allows you to fight off a cold, for example).xxvi
Other theories on resistance propose that some species acquire resistance through the bacteria and toxins on their skin. The skin on certain amphibian species harbors bacteria that fight off Bd. All amphibians coat their skin in toxins, some of which may prevent the growth of Bd. These ideas are still under investigation and have not yet provided any useable conclusions.xxvii
Behavior can also factor into resistance. Amphibians that inhabit warmer and drier areas are less likely to succumb to infection. Bd zoospores easily swim through water, but cannot spread as quickly in dry areas. The fungus also does best in mild climates, so amphibians that spend more time in warm to hot places are less susceptible to Bd.xxiii
Disease Origin
Scientists have found it hard to identify the origin of the Bd. North America, Africa, Asia, and Brazil have all been proposed as where Bd originated. Because of this, it is possible that the current outbreak originated from multiple centers. The amphibian trade then brought these strains into new areas where the amphibians had not encountered Bd before. These “naïve” amphibian populations are the ones with the highest mortality rates during the current outbreak. Although Bd is only known to reproduce asexually, some recombinant strains of Bd have been identified. This indicates that some sexual reproduction has occurred. The current outbreak thus has a complex genetic history, which makes it harder to combat the disease.xxix

The population of fire salamanders in Europe crashed after Bsal was introduced. Public Domain, via Wikimedia
Bsal has a much simpler backstory. That pathogen is endemic in Asia, where it causes non-lethal infections in salamanders and newts. The pet trade was responsible for introducing the disease into Europe, where it quickly spread among naïve salamander populations. In laboratory experiments, most North American salamanders and newts do not show much resistance to the Bsal. This suggests the pathogen will spread quickly if it ever makes it to North America.xxx
Solutions
Unfortunately, not much can be done to mitigate the impact of chytridiomycosis in the wild. There are treatments available for pets, but these would not work on a large scale.xxxi Management in the wild focuses on preserving as much of the population as possible. One possible strategy is to extract a portion of an amphibian population and maintain the animals in captivity to preserve their genes. Another strategy is to support existing populations by improving their native habitat. This should help species be more resilient to chytridiomycosis. Additionally, captive species can be reintroduced to the wild to support struggling populations.xxxii One further idea to reduce the incidence of chytridiomycosis is to use natural predators of Bd. For example, zooplankton eat Bd zoospores and therefore could be used to reduce the number of spores encountered by amphibians.xxxiii Bsal chytridiomycosis also can be mitigated by preventing any further spread of the pathogen. This can be done through awareness and by not importing Asian salamanders to uninfected areas.xxxiv
However, there is some hope that amphibian populations can recover naturally. As mentioned above, some species are recovering despite chytridiomycosis.xxxv Additionally, some species have small “persistent populations,” that remain small but are infected at lower rates. The process behind these persistent populations is not understood.xxxvi
Of course, despite the severity of chytridiomycosis, disease is not the greatest threat to amphibians. That title belongs to habitat loss, which is mostly driven by human activity.xxxvii Thus, ensuring that amphibians have suitable habitat is an important method for helping them recover.
See Further:
http://www.amphibianark.org/the-crisis/chytrid-fungus/
http://www.salamanderfungus.org/about-bsal/
http://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5423370.pdf
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4660679/
http://www.bbc.com/news/science-environment-19199197
Citations
[i] Lee Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians,” Fungal Ecology 19 (February 1, 2016): 89–99, doi:10.1016/j.funeco.2015.09.007; Rick Speare, Lee Berger, and Keith McDonald, “Background Document for the Threat Abatement Plan: Infection of Amphibians With Chytrid Fungus Resulting in Chytridiomycosis” (Australian Government Department of the Environment and Heritage, 2006), http://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5423370.pdf.
[ii] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[iii] Allan Pessier, “Chytrid Fungus,” Amphibian Ark, accessed September 7, 2016, http://www.amphibianark.org/the-crisis/chytrid-fungus/.
[iv] “Chytridiomycosis (Amphibian Chytrid Fungus Disease)” (Australian Government Department of Sustainability, Environment, Water, Population and Communities), accessed September 7, 2016, https://www.environment.gov.au/biodiversity/invasive-species/publications/factsheet-chytridiomycosis-amphibian-chytrid-fungus-disease.
[v] Pessier, “Chytrid Fungus.”
[vi] Pascale Van Rooij et al., “Amphibian Chytridiomycosis: A Review with Focus on Fungus-Host Interactions,” Veterinary Research 46 (2015), doi:10.1186/s13567-015-0266-0.
[vii] Salamanderfungus. org, “What Is Bsal?,” Bsal Task Force, accessed September 8, 2016, http://www.salamanderfungus.org/about-bsal/.
[viii] Pessier, “Chytrid Fungus.”
[ix] Salamanderfungus. org, “What Is Bsal?”
[x] Van Rooij et al., “Amphibian Chytridiomycosis.”
[xi] Pascale Van Rooij et al., “Germ Tube Mediated Invasion of Batrachochytrium Dendrobatidis in Amphibian Skin Is Host Dependent,” PloS One 7, no. 7 (2012): e41481, doi:10.1371/journal.pone.0041481.
[xii] Van Rooij et al., “Amphibian Chytridiomycosis.”
[xiii] Speare, Berger, and McDonald, “Background Document for the Threat Abatement Plan: Infection of Amphibians With Chytrid Fungus Resulting in Chytridiomycosis.”
[xiv] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xv] Salamanderfungus. org, “What Is Bsal?”
[xvi] Speare, Berger, and McDonald, “Background Document for the Threat Abatement Plan: Infection of Amphibians With Chytrid Fungus Resulting in Chytridiomycosis.”
[xvii] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xviii] Van Rooij et al., “Amphibian Chytridiomycosis.”
[xix] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xx] Richard Black, “Climate Change May Boost Frog Disease Chytridiomycosis,” BBC News, August 12, 2012, sec. Science & Environment, http://www.bbc.com/news/science-environment-19199197.
[xxi] “Chytridiomycosis (Amphibian Chytrid Fungus Disease).”
[xxii] Van Rooij et al., “Amphibian Chytridiomycosis.”
[xxiii] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xxiv] Pessier, “Chytrid Fungus.”
[xxv] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xxvi] Speare, Berger, and McDonald, “Background Document for the Threat Abatement Plan: Infection of Amphibians With Chytrid Fungus Resulting in Chytridiomycosis.”
[xxvii] Pessier, “Chytrid Fungus.”
[xxviii] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xxix] Van Rooij et al., “Amphibian Chytridiomycosis.”
[xxx] Salamanderfungus. org, “What Is Bsal?”
[xxxi] Pessier, “Chytrid Fungus.”
[xxxii] Speare, Berger, and McDonald, “Background Document for the Threat Abatement Plan: Infection of Amphibians With Chytrid Fungus Resulting in Chytridiomycosis.”
[xxxiii] Berger et al., “History and Recent Progress on Chytridiomycosis in Amphibians.”
[xxxiv] Salamanderfungus. org, “What Is Bsal?”
[xxxv] Speare, Berger, and McDonald, “Background Document for the Threat Abatement Plan: Infection of Amphibians With Chytrid Fungus Resulting in Chytridiomycosis.”
[xxxvi] Pessier, “Chytrid Fungus.”
[xxxvii] Ibid.







![#011: Characteristics of Kingdom Fungi [Archived]](https://www.fungusfactfriday.com/wp-content/themes/hueman/assets/front/img/thumb-small-empty.png)

3 Responses
[…] and microscopic worms to large plants and amphibians. You may have heard about chytridiomycosis (FFF#157), which is killing millions of frogs worldwide. This disease is caused by the chytrid […]
[…] interfering with their skin, which is essential for proper regulation of frog body chemistry (see FFF#157 for more). Thankfully, some frogs appear to be developing a resistance to the fungus. […]
[…] disease chytridiomycosis (Batrachochytrium dendrobatidis, FFF#157) has killed innumerable amphibians (especially frogs) across the globe. Many efforts are […]