#195: Warfarin, from Cow Disease to Medicine

Sweet clover (Melilotus alba – above – and M. officinalis) is a common hay used to feed cattle in northern North America. Unfortunately, it also contains coumarin which fungi convert into a toxic compound when given the chance. Public domain image.
Warfarin is one of the most successful drugs of all time. Seventy years after it was first synthesized, warfarin is still the most widely prescribed anticoagulant. Warfarin has a unique story. What began as depression-era research into a mysterious disease of cattle ended up producing two life-saving medicines and a rat poison that are still in use today.1
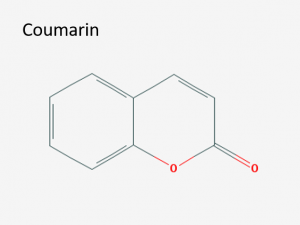
Structure of coumarin (IUPAC name: chromen-2-one). Image via PubChem.
Coumarin
Our story begins with the molecule coumarin. Coumarin is a secondary metabolite produced by a variety of plants.2,3 It is mildly toxic and carcinogenic, although humans can safely ingest up to 0.1mg per kilogram of body weight every day before it causes any damage. For humans, the most common food containing coumarin is cinnamon. Fortunately, normal use of cinnamon delivers a dose of coumarin well below the daily tolerance limit (heavy use of cinnamon may put some people over the limit).2
For the story of warfarin, however, sweet clover (Melilotus alba and M. officinalis) is the most important source of coumarin. Sweet clover is grown for use as green manure and hay to feed cattle, primarily in northern North America. The amount of coumarin found in sweet clover is too low to harm cattle and most of the time its presence goes completely undetected.1,4
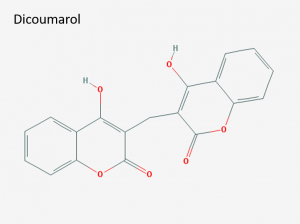
Structure of dicoumarol (IUPAC name: 4-hydroxy-3-[(4-hydroxy-2-oxochromen-3-yl)methyl]chromen-2-one). Image via PubChem.
Dicoumarol
In the 1920’s, the United States experienced a string of wet summers that brought along a mysterious cattle disease. Suddenly, there was an epidemic of cattle dying of internal bleeding.5 The disease was particularly troublesome because the cattle did not have any symptoms until the disease was already severe. Symptoms included weakness, swelling under the skin (indicative of pooled blood), pale mucous membranes, and excessive internal and external bleeding.4 Scientists at the time struggled to find a cause, ruling out both nutritional causes and infectious diseases. Eventually, they examined the diet of the cattle and discovered that all the infected animals had been fed sweet clover. After some more research, the cause of the disease was found to be moldy sweet clover.1 The offending hay always hosted a species of mold, typically Penicillium nigricans, P. jensi, or Aspergillus spp. This led to an easy fix for the hemorrhagic disease: stop feeding the animals moldy clover.1,4
Soon after this, the Great Depression struck and many farmers were forced to feed their livestock moldy hay. In 1933, one of these farmers who upset at continually losing cattle to the disease took his problem to Karl Paul Link, a local agricultural scientist. Helpfully, the farmer brought along 100 pounds of moldy sweet clover and a canister of uncoagulated cow blood. After six years of carefully extracting compounds from spoiled hay, Link and his colleagues (especially Link’s student Mark A. Stahmann) managed to isolate a single compound responsible for the “sweet clover disease.” This compound had the structure 3,3’-methylene-bis[4-hyfroxycoumarin] and Link’s team named it dicoumarol.1,5 Soon after, clinical trials began at the Mayo Clinic to test dicoumarol’s safety and efficacy as an anticoagulant. Dicoumarol passed these trials and first became available to a national audience in 1941.5
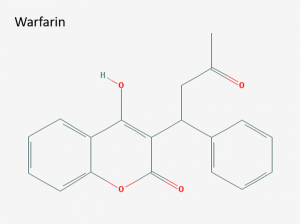
Structure of warfarin (IUPAC name: 4-hydroxy-3-(3-oxo-1-phenylbutyl)chromen-2-one). Image via PubChem.
Warfarin
Once you’ve isolated and know the structure of a useful molecule, the next step is to check to see if any derivatives of that compound are biologically active as well. Link and his colleagues tested 150 different derivatives and found one that was more potent than dicoumarol. They named this compound warfarin after the Wisconsin Alumni Research Foundation, which had funded most of the research on dicoumarol and warfarin. Because warfarin was so potent, it was originally marketed as a very successful rat poison. However, doctors eventually realized that warfarin was easier to use than dicoumarol. Warfarin is very soluble in water and is easily taken up by the intestines. This means that warfarin can be easily stored in pills and when it is ingested nearly all the medicine goes into the blood stream. Thanks to these properties, warfarin could be used as an oral pill with much less hassle than dicoumarol. Once people accepted that a rat poison could also be medicine, warfarin (under the brand name “Coumadin”) went on to become the most popular anticoagulant.1,5
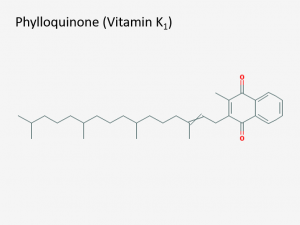
Structure of vitamin K1, a.k.a. phylloquinone (IUPAC name: 2-methyl-3-(3,7,11,15-tetramethylhexadec-2-enyl)naphthalene-1,4-dione). Both warfarin and dicoumarol are structurally similar to vitamin K, which allows them to interrupt clotting factor synthesis. Image via PubChem.
Mechanism of Action
Warfarin and dicoumarol are similar in structure and therefore act in similar ways. Both compounds have a chemical structure reminiscent of vitamin K. Thanks to this similarity, the compounds have the ability to block the production of “vitamin K dependent coagulation factors.” These factors are various components of blood plasma that are required for blood to congeal properly. They all use vitamin K as a component of their final structures, so preventing them from biding to vitamin K stops the formation of any new vitamin K dependent coagulation factors. Because warfarin and dicoumarol are similar in structure to vitamin K, they can attach to the various protein complexes that build the coagulation factors and either prevent those proteins from working or prevent them from replenishing the cell’s supply of vitamin K.6
See Further:
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2141.2008.07119.x/pdf
http://www.jbc.org/content/280/8/e5
https://www.drugs.com/pro/warfarin.html
http://poisonousplants.ansci.cornell.edu/toxicagents/coumarin.html
http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/faq8154?opendocument
Citations
- Wardrop, D. & Keeling, D. The story of the discovery of heparin and warfarin. British Journal of Haematology 141, 757–763 (2008).
- Abraham, K., Wöhrlin, F., Lindtner, O., Heinemeyer, G. & Lampen, A. Toxicology and risk assessment of coumarin: focus on human data. Mol Nutr Food Res 54, 228–239 (2010).
- Coumarin. National Center for Biotechnology Information, PubChem Compound Database Available at: https://pubchem.ncbi.nlm.nih.gov/compound/323. (Accessed: 18th August 2017)
- Coumarin Glycosides. Cornell University College of Agricultural and Life Sciences, Department of Animal Science – Plants Poisonous to Livestock (2015). Available at: http://poisonousplants.ansci.cornell.edu/toxicagents/coumarin.html. (Accessed: 18th August 2017)
- Kresge, N., Simoni, R. D. & Hill, R. L. Hemorrhagic Sweet Clover Disease, Dicumarol, and Warfarin: the Work of Karl Paul Link. J. Biol. Chem. 280, e5–e5 (2005).
- Warfarin. Drugs.com (2017). Available at: https://www.drugs.com/pro/warfarin.html. (Accessed: 18th August 2017)







![#011: Characteristics of Kingdom Fungi [Archived]](https://www.fungusfactfriday.com/wp-content/themes/hueman/assets/front/img/thumb-small-empty.png)